Shengshi Taikang Opens the Door to Capital Markets, Injecting New Momentum into Innovative Drug Development
Release date:
2025-09-29

, marking the company's official entry into the capital market to support the commercialization of its listed products and accelerate the advancement of its clinical pipeline. (Security Abbreviation: Shengshi Taikang, Security Code: 874899) , marking the company's official entry into the capital market to support the commercialization of its上市 products and accelerate the advancement of its clinical pipeline.

As a biotechnology company in the commercialization phase, Shengshi Taikang's core team boasts decades of international experience across the entire lifecycle of pharmaceutical products. The company is dedicated to the research, development, and industrialization of high-quality, differentiated innovative drugs. Leveraging an integrated drug R&D technology platform and a diversified business perspective, Shengshi Taikang has built a robust pipeline of cutting-edge therapies spanning areas such as blood sugar control, cancer treatment, and autoimmune diseases—enabling a positive "R&D-to-market-to-pipeline expansion" cycle.
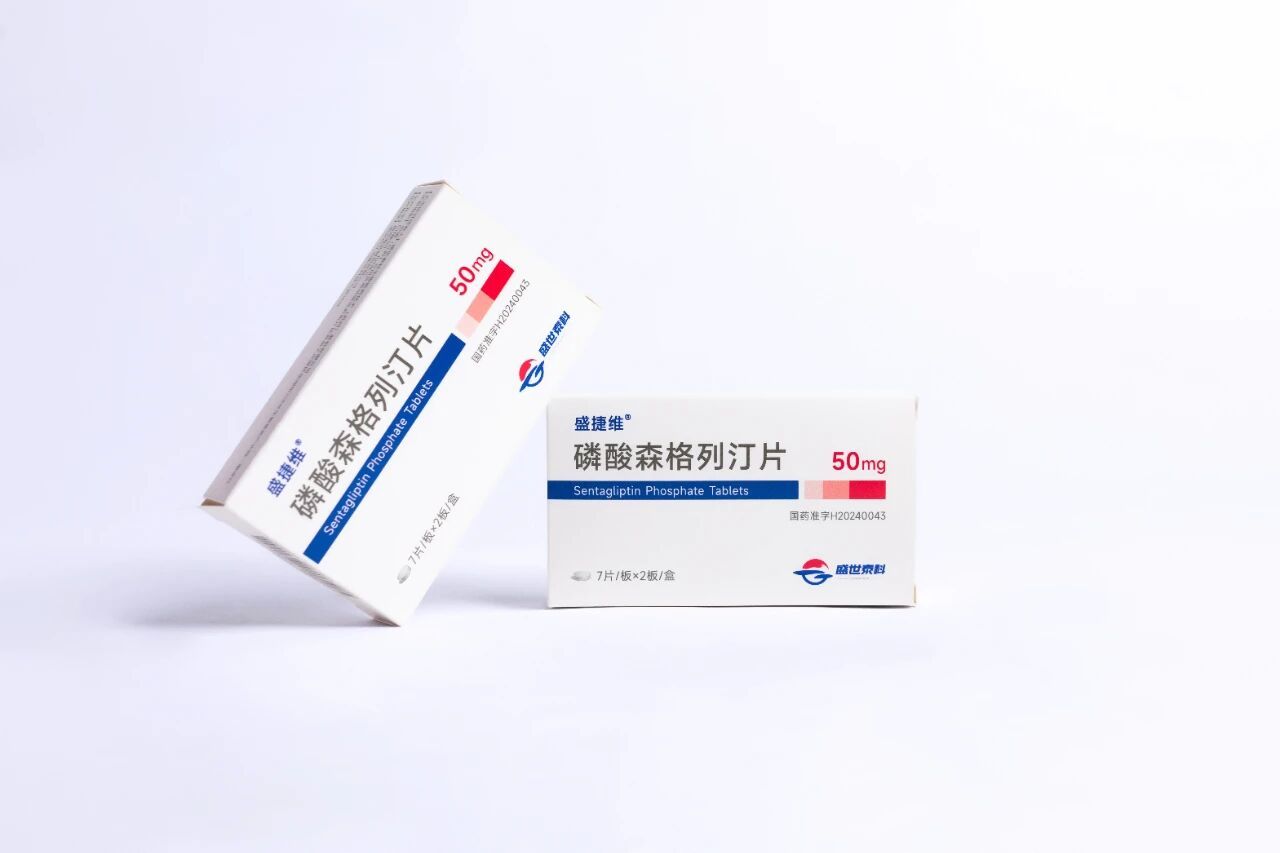
Currently, Company's Class 1 Innovative Drug: Sen Gleeting (Product name: Shengjiewei) has been successfully launched and is now available for sale. , becoming the core engine driving its commercial operations. This next-generation, highly selective DPP-4 inhibitor—specifically designed for type 2 diabetes—not only earned its place in both the National 12th Five-Year Plan and the 13th Five-Year Plan’s “Major New Drug Development” initiatives but also demonstrated remarkable "half-dose, double-effect" advantages during Phase 3 clinical trials. At low doses, it successfully achieved the pre-specified trial endpoints, while the higher-dose group further highlighted the drug’s excellent safety profile—positionsing it as a potential best-in-class glucose-lowering therapy. Meanwhile, the company has strategically expanded its portfolio around globally recognized diabetes targets, developing comprehensive solutions that encompass both oral medications and treatments for diabetes-related complications, ultimately enhancing its ability to address diabetes and its associated conditions more effectively.
In another key area of the company's R&D pipeline—specifically, the field of anti-cancer drugs— Shengshi Taikе Phase breakthroughs have been achieved, with four innovative drugs entering clinical trials. Among them, CXCR4 antagonists are increasingly gaining attention worldwide, and CGT-1881, targeting this specific pathway, is being explored in therapeutic areas such as hematopoietic stem cell mobilization. It is the only domestically developed, original small-molecule oral CXCR4 antagonist in China. , making drug administration more convenient. Current clinical trial data indicate that it effectively promotes stem cell mobilization and WHIM We have very strong confidence in the syndromes. Among the four oncology clinical products, two projects have already received U.S. FDA clinical approval, marking a solid step forward for the company’s innovative drugs as they head overseas. Additionally, the company has developed its first domestically produced treatment for multiple sclerosis, a rare disease. Treliflumine Tablets Now available for sale.
Shengshi Taikang has successfully listed on the New Third Board, laying a solid foundation for future transfers and alignment with higher-tier capital markets, while continuously strengthening its "capital-driven innovation" capabilities in R&D. Moving forward, the company will remain focused on unmet clinical needs, developing innovative drugs that are highly accessible and deliver superior therapeutic outcomes—ultimately benefiting patients worldwide.
Prev:
