The "2018 Top 100 Future Healthcare Companies" list by Arterial Network has been officially released, and Shengshi Taikang is proudly featured on the list.
Release date:
2018-12-20
From December 18 to 19, 2018, the Arterial Network "2018 Top 100 Future Healthcare" Forum was grandly held at the Ritz-Carlton Hotel in Beijing. Over 2,500 health and medical industry leaders from home and abroad attended the prestigious event. 
On the 18th, Arterial Network proudly unveiled its four flagship lists for the "2018 Future Healthcare Top 100": the "China Healthcare Top 100," the "China Pharmaceutical Top 100," the "China Health Top 30," and the "Overseas Top 100." We are delighted that Shengshi Taikang has earned a spot among the top 100 on the China Pharmaceutical List.
Additionally, since 2018, Arterial Network has introduced sub-lists (TOP5) covering 12 specialized fields such as rehabilitation robots, DTP pharmacies, and third-party healthcare services, along with the "Top 10 Chinese Industrial Park Rankings" and the "Top 10 Chinese New Industry City Rankings."
Living in a great era defined by the exploration of tomorrow's "trends" in healthcare, we look forward to groundbreaking technological advancements and innovations, as well as pioneering models and improvements—all poised to deliver even more exciting possibilities for the healthy future of humanity.
As an observer and chronicler of the industry, Arterial Network aims to examine societal transformations, economic fluctuations, and evolving consumer trends—from both a Chinese and global perspective—enabling a broader understanding of how the health-care industry is reshaping its trajectory. The "Top 100 Future Healthcare Companies" initiative seeks to identify China's most innovative healthcare enterprises that truly embody the future of medicine, uncovering the core drivers of our nation’s upcoming healthcare industry and accelerating the momentum behind transformative innovation in the sector.
In 2018, the "Top 100 Future Healthcare Companies" list was meticulously curated through three rigorous stages—initial selection, review, and final approval—to identify the most promising enterprises across various sub-sectors of the healthcare industry. The list serves as a benchmark for innovative companies to follow, provides valuable guidance for investment firms, and delivers tangible value to its audience.
Here is the list of companies featured in the 2018 "Top 100 Future Healthcare" China Healthcare Ranking:
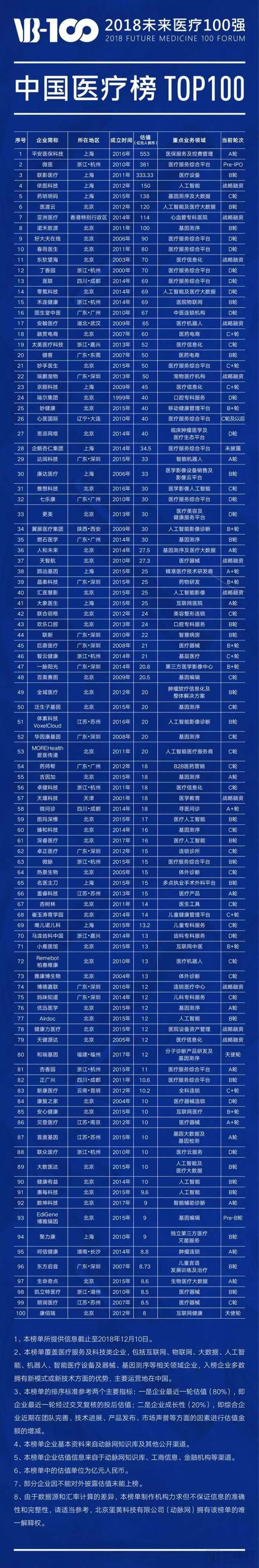
Here is the list of companies featured in the 2018 "Top 100 Chinese Healthcare Innovators" ranking:
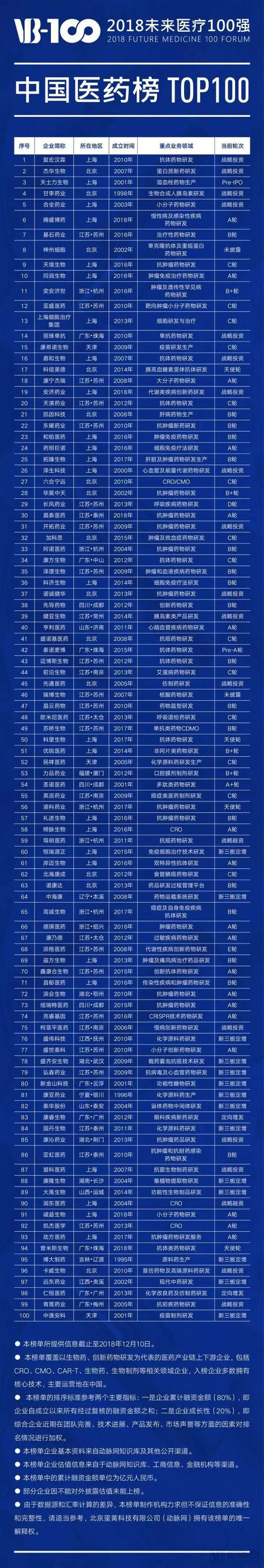
Here is the list of companies featured in the 2018 "Top 100 Future Healthcare" China Health Ranking:
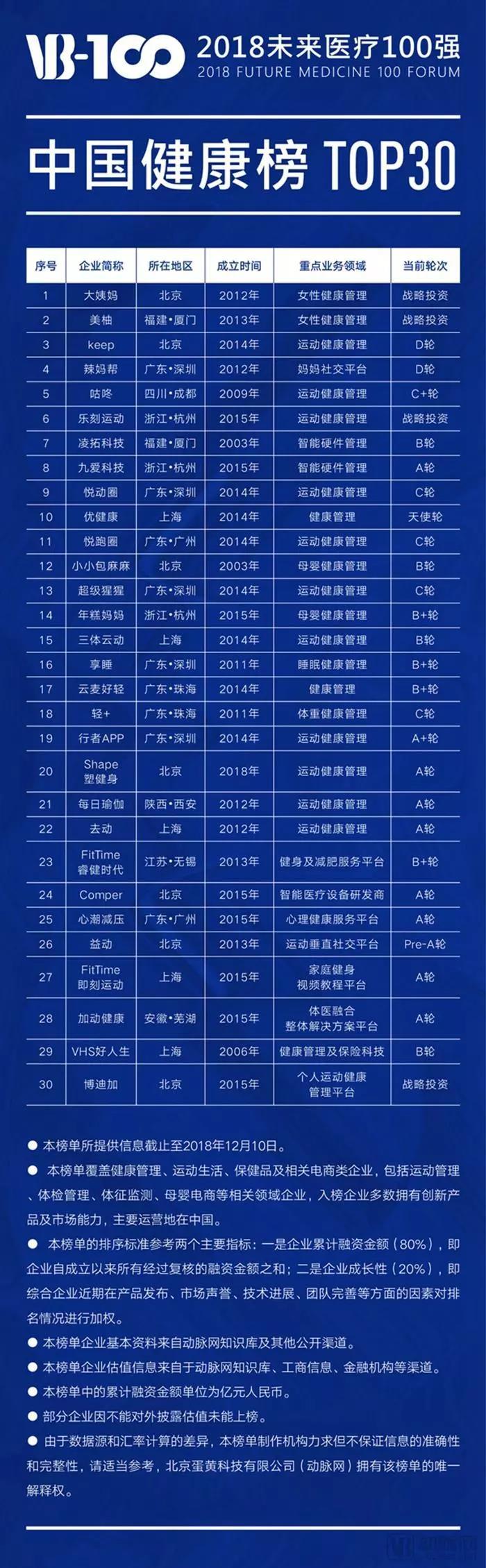
Here is the list of companies featured in the 2018 "Top 100 Global Healthcare Innovators" overseas ranking:
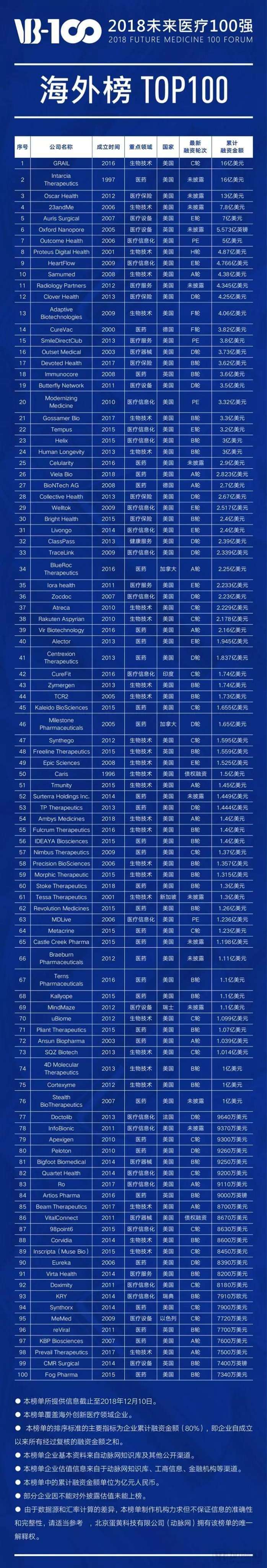
The "2018 Future Healthcare Industry Report," released by Arterial Network and Eggshell Research Institute, highlights that 2018 marked a transformative year for the healthcare sector, as China achieved significant progress in its ongoing reforms. Notably, healthcare service capabilities were strengthened, public health services saw notable improvements, hospital management became increasingly standardized, and insurance coverage continued to expand. Meanwhile, regulatory bodies have stepped up their support for innovative pharmaceuticals, while groundbreaking medical technologies have emerged one after another.
Driven by a variety of factors, several standout companies have emerged across diverse segments of healthcare innovation—ranging from established firms eager to embrace bold new changes to pioneering startups charging ahead with unwavering momentum. These companies have become the most influential players in the ongoing journey of medical and healthcare innovation.
The "Top 100 Future Healthcare Companies" 2018 annual awards were determined through a public voting process conducted by an expert panel comprising industry professionals, investors, and policy researchers. The winners were jointly selected by the Arterial Network Content Center, the Eggshell Research Institute, and the industry judges, ultimately resulting in several prestigious annual accolades, including sub-lists highlighting specific segments of the "Future Healthcare 100," as well as city and park-based rankings.
The sub-lists within the specialized fields include "Rehabilitation Robots," "DTP Pharmacies," "Third-Party Medical Services," "Medical IoT," "Pediatric Clinics," "Physician Groups," "Digital Traditional Chinese Medicine," "Pharmacy Digitalization Equipment," "Insurance Payments," "Consumer Genetics," "Gene Editing," and "Microbiome"—a total of 12 key sub-lists. The list is as follows: :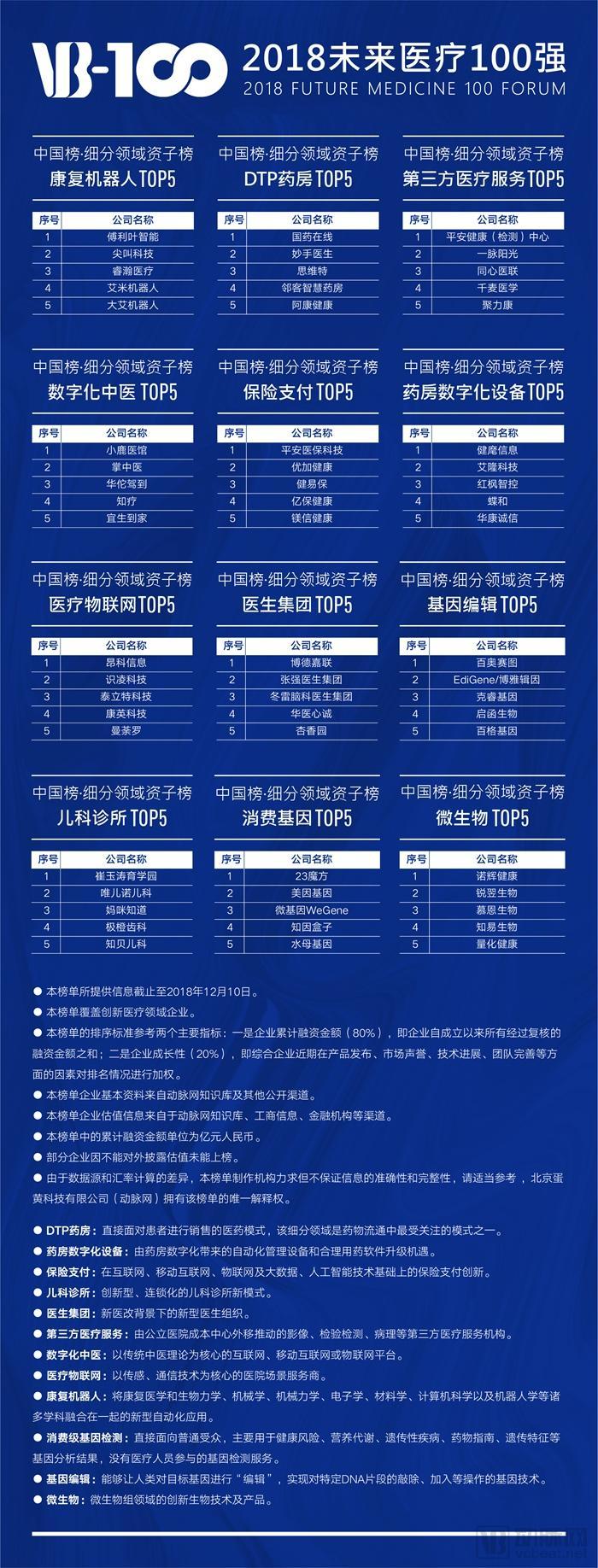
The 2018 Top 100 Chinese Innovators in Future Healthcare — List of Industrial Park and New Industry City Rankings is as follows:
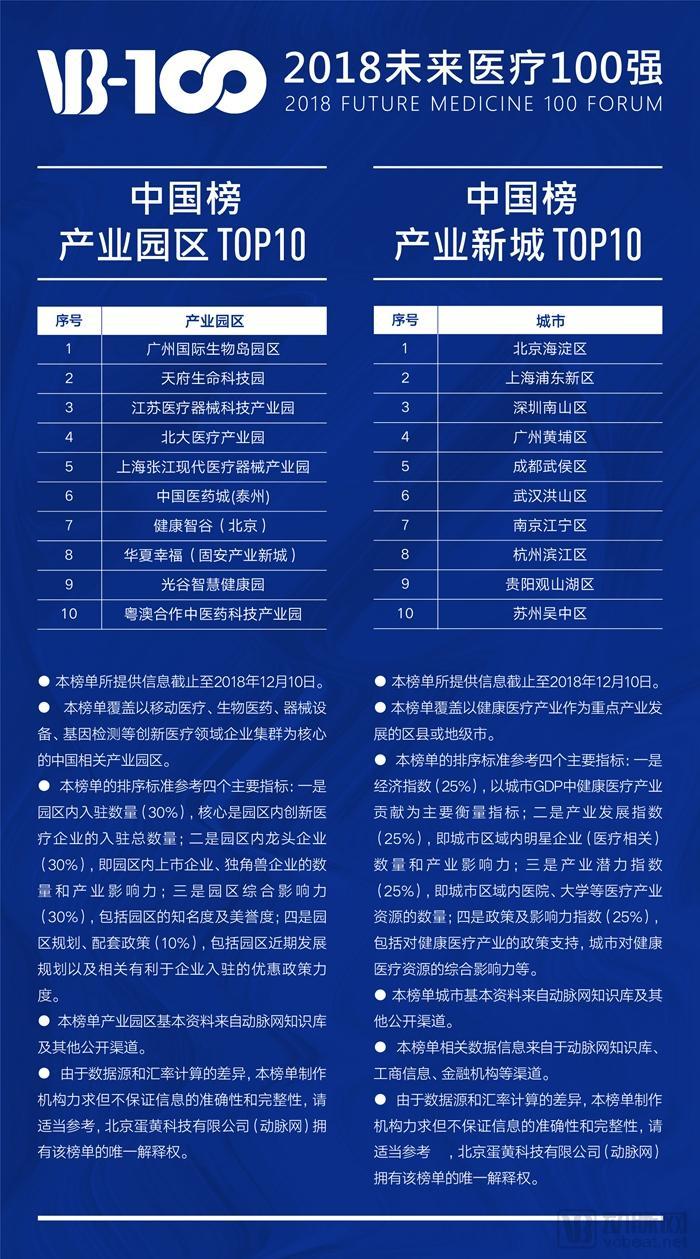
Source: Arterial Network
