Brainstorming | Setting Sail Through Metabolism, Exploring the Stars and the Sea
Release date:
2024-05-28

With the advancement of the "Healthy China 2030 Planning Outline," chronic disease medications have become a key focus in pharmaceutical R&D. Among these, the development of cardiovascular, lipid-lowering, and blood sugar- and weight-management drugs has particularly emerged as a hot trend in the industry. Recently, ResearcherTalk and Haomai Technology hosted a "brainstorming session" in Zhuhai, centering on clinical needs, industry trends, and the latest advancements in metabolic disease drug development. Dr. Yu Qiang, Founder and CEO of Shengshitaike Innovation, attended the event and joined guests in exploring the vast potential of chronic disease therapies—from a metabolic perspective—toward new frontiers in medicine.

Ms. Gong Yuanxiang, Founder of Haomai Technology
Professor Liu Dongyang: Challenges in Developing New Drugs for Glycemic Control and Metabolic Diseases Using Mathematical Models
Researcher Liu Dongyang, Deputy Director of the Clinical Trial Institution at Peking University Third Hospital, presented insights into the challenges of clinical research guided by quantitative pharmacology models in diabetes drug development, as well as innovative strategies leveraging digital models to accelerate the development of novel glucose-lowering therapies. He also discussed the advancements and hurdles in using digital approaches for diabetes treatment. Professor Liu further explained the definition and role of quantitative pharmacology: to enhance both the efficiency and quality of innovative drug development, while simultaneously advancing precision medicine research.

Professor Liu Dongyang, Deputy Director of the Clinical Trial Unit at Peking University Third Hospital
He suggested that research based on pharmacokinetic-pharmacodynamic virtual humans could become a groundbreaking new model for future drug development, with quantitative pharmacology serving as its foundation. He emphasized that the central challenge in quantitative pharmacology lies in enhancing the predictive power of models to enable accurate forecasting.
Professor Liu specifically noted that in recent years, both the U.S. FDA and China’s National Medical Products Administration’s Center for Drug Evaluation have strongly promoted MiDD—“Model-informed Drug Development”—a transformative approach to new drug research and development. At the heart of MiDD lies the principle of building PK-PD models based on correlations between blood drug concentrations (i.e., exposure, PK) and therapeutic efficacy/safety outcomes (i.e., effect, PD). These models are then used to simulate study conditions tailored to specific research objectives, enabling researchers to analyze the exposure-effect relationships in both general and subgroup populations—thus substituting for the often challenging-to-obtain dose-effect data. This process ultimately helps establish a robust evidence chain linking a drug’s efficacy and safety.
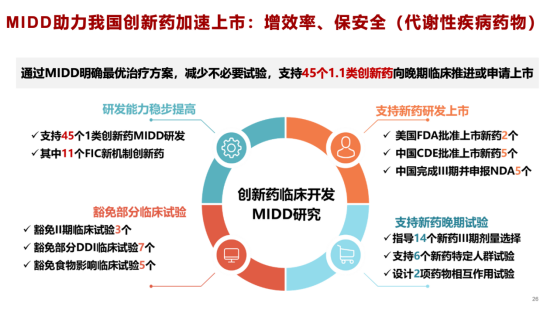
Meanwhile, in the field of diabetes research, Professor Liu’s team has already developed first- and second-generation virtual human models for pharmacokinetics of diabetes drugs. Building on innovative approaches that draw from foreign models—specifically those based on islet cell experiments used to predict human glucose responses during oral glucose tolerance tests and prediabetes assessments—they have created a sophisticated modeling method that accurately estimates insulin secretion capacity and sensitivity using data from glucose tolerance tests. Looking ahead, Professor Liu anticipates that the next decade will usher in the era of personalized medicine, emphasizing the critical role of clinical decision-support software in optimizing individualized drug therapies. Such tools, he believes, will not only enhance treatment efficacy but also play a vital role in extending patients' lives after new medications are approved for market.
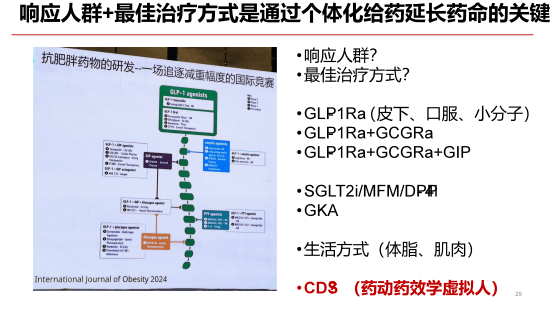
Dr. Yu Qiang: Setting Sail from Metabolism to Explore the Vast Cosmos
Dr. Yu Qiang, CEO of Shengshi Taikang, boasts 30 years of experience in small-molecule drug research and development. After graduating from Peking University’s Chemistry Department in his early years, he pursued advanced studies in the United States. In 2010, Dr. Yu made the courageous decision to return to China and founded an innovative biopharmaceutical company focused on developing metabolic and oncology drugs. Speaking about the promising future of metabolic therapies, Dr. Yu expressed unwavering confidence, vividly comparing the market to a vast, boundless sea of stars—full of limitless potential and hope.
Dr. Yu Qiang, CEO of Shengshi Taikang
He highlighted the continued growth of the global diabetes market, which is projected to surpass $1 trillion by 2030. According to data from the International Diabetes Federation (IDF), in 2021, there were 536.6 million adults worldwide living with diabetes—representing a prevalence rate of 10.5%. Forecasts suggest that by 2030, the number of adult diabetes patients globally will rise to 642.7 million, an increase of 20%. In 2021, global healthcare spending on diabetes reached approximately $966 billion, and it’s expected that total diabetes-related medical expenditures will exceed $1 trillion by 2030. Meanwhile, as Chinese lifestyles continue to evolve, diabetes is set to become a major public health challenge.
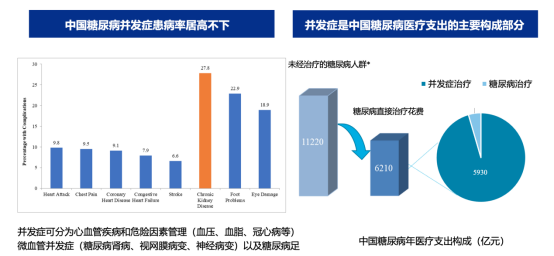
China is the country with the largest number of diabetes cases in the world. In 2021, the country had approximately 140 million adult diabetes patients, ranking first globally. However, rather than focusing solely on blood sugar management, preventing, delaying, and controlling diabetes-related complications should be the top priority in diabetes treatment. Chronic complications of diabetes include macrovascular diseases, microvascular complications, peripheral neuropathy, and autonomic neuropathy.
Take large-vessel disease as an example: diabetic macrovascular complications primarily affect blood vessels in vital organs such as the heart and brain, with common complications including myocardial infarction and stroke. Beyond their blood-glucose-lowering effects, antidiabetic drugs that also offer significant organ-protective benefits will likely become even more favored by clinicians.
In fact, the development of diabetes treatments has a long and rich history. Dr. Yu explained the mechanism of action behind DPP-4 inhibitors, as well as clinical studies comparing different options for diabetes medication and their respective benefits. Currently, DPP-4 inhibitors—including linagliptin, vildagliptin, saxagliptin, alogliptin, and sitagliptin—offer varying oral bioavailability rates ranging from 30% to 100%.
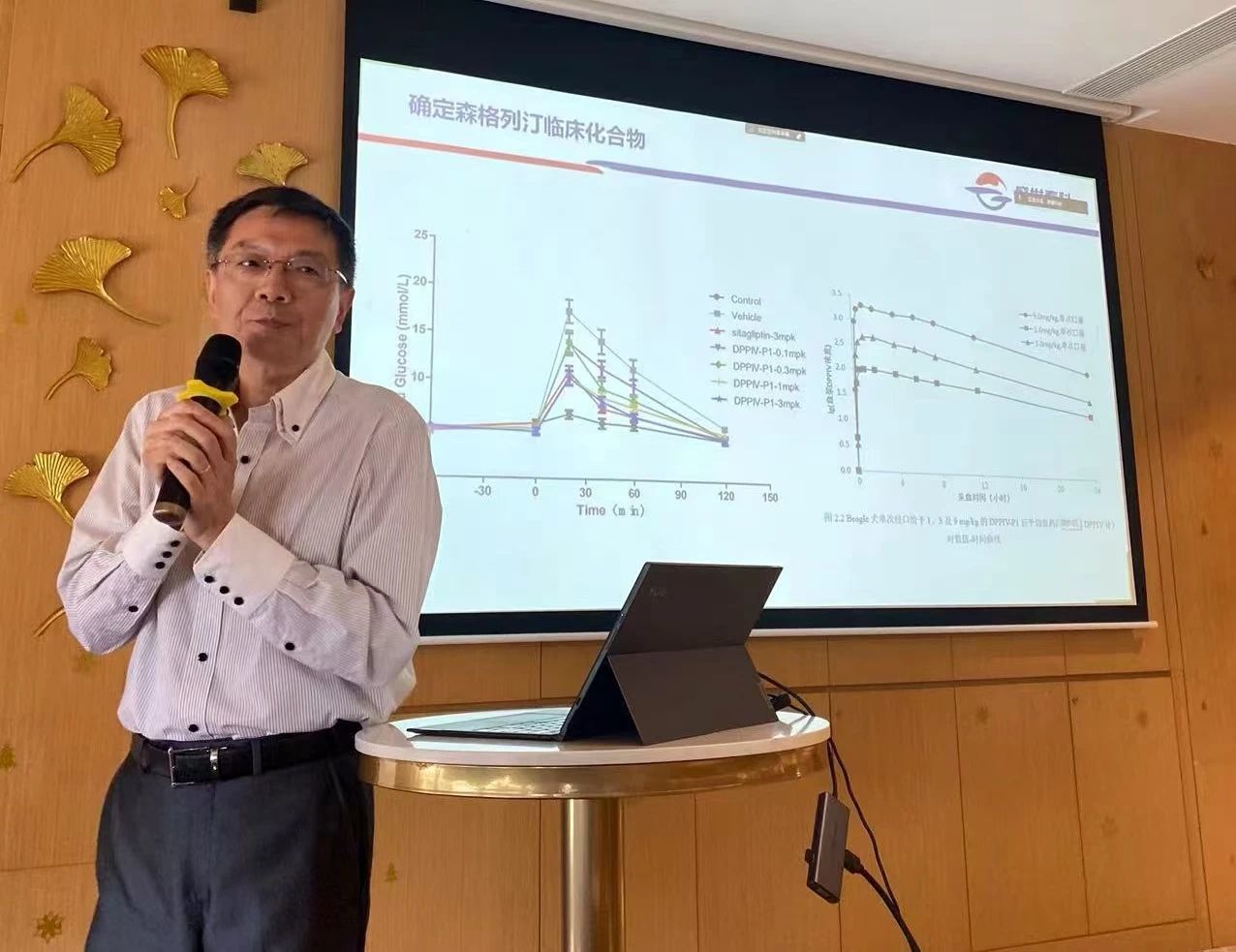
He introduced the development background and strategic positioning of Senglidiene (formerly known as Shenglidiene), an innovative Class 1 drug independently developed by Shengshi Taikang. According to the FBDD theory, many drug targets have active pockets composed of multiple sub-active cavities, enabling drugs to achieve optimal binding with their targets. Senglidiene was specifically designed based on this principle, ensuring robust target engagement with high selectivity and specificity. Preclinical study results show that Senglidiene outperforms the current global benchmark product in its class, a finding that has brought immense joy to both him and domestic researchers.
In July 2019, Senglitazone pioneered the first quantitative pharmacology model in the DPP-4 inhibitor field, injecting fresh momentum into the development of novel drug clinical research. Using this model, researchers precisely estimated changes in HbA1c (glycated hemoglobin) after 12 and 24 weeks of treatment, enabling them to scientifically predict the optimal dosage of Senglitazone for its Phase 3 clinical trials. As a result, Senglitazone became the first drug to directly advance from Phase 2 to Phase 3 clinical testing via a quantitative pharmacology model. Meanwhile, with the growing prevalence of co-existing conditions such as hyperglycemia, hypertension, and hyperlipidemia, the trend toward developing combination therapies is becoming increasingly evident—and Senglitazone holds significant potential for application in this promising area.
Dr. Xiao Shen: Cardiovascular 、 Clinical Development and Regulatory Pathways for New Drugs Targeting Kidney and Metabolic Diseases
Dr. Xiao Shen, Chief Medical Officer of Shanghai Haishen Biopharmaceutical, presented a discussion on the clinical development and regulatory pathways for new drugs targeting cardiovascular, renal, and metabolic diseases.

Dr. Xiao Shen, Chief Medical Officer of Haisen Biopharmaceutical
Yu Zhong also analyzed the R&D trends of GLP-1 class drugs from the perspective of patent big data. China has experienced a rapid surge in GLP-1 patents since 2020, surpassing the U.S. by 2023. From a formulation standpoint, both oral and injectable GLP-1 patent applications have their own distinct strengths. In terms of target combinations, GLP-1/GIP, GLP-1/GCG, and GLP-1/GCG/GIP continue to maintain high levels of patent activity, while GLP-1/PYY and GLP-1/secretin have seen significant growth in recent years, making them particularly noteworthy. Regarding institutional patent filings, Gasherbrum Bio appears to be a promising new entrant among players that have entered the market in recent years.
Additionally, Xiao Shen also discussed the considerations for selecting endpoints in clinical trials of drugs for metabolic diseases, including preliminary proof-of-concept (POC) assessments, identification of primary efficacy endpoints, clinical endpoints, surrogate endpoints, composite endpoints, patient-reported outcomes, and the use of biomarkers.
Dr. Xiao Shen, while discussing cases of cardiovascular and kidney disease drug approvals, emphasized the FDA's rigorous standards for evaluating new medications. He highlighted four examples where FDA-approved drugs were ultimately rejected, underscoring that the agency carefully weighs a drug’s risks against its benefits—and is especially stringent when it comes to the safety of chronic-disease treatments. During the approval process, the FDA places significant emphasis on robust safety data, as well as clinical endpoints that directly reflect improvements in patients' overall clinical quality of life. Additionally, the FDA conducts thorough comparisons with existing therapies in the same class. When developing new drugs, it’s crucial to thoroughly account for patient-specific factors and potential drug interactions, while also selecting appropriate clinical endpoints to accurately assess both efficacy and safety.
Yu Zhong: From a big data perspective G LP-1 Research Focus Areas
Yu Zhong, co-founder of Haomai Technology, stated that GLP-1, as a class of drugs used to treat diabetes and obesity, currently faces significant challenges in clinical practice. Notably, high discontinuation rates due to side effects—nearly two-thirds of patients stop taking the medication within one year—are a major issue. Moreover, after stopping treatment, patients often experience rapid weight regain, while also facing a concerning decline in lean body mass (the amount of muscle and other metabolically active tissue) by as much as 37% during weight loss. Additionally, diminishing weight-loss effectiveness after two years, coupled with the inconvenience of injection-based administration, further restricts the broader adoption of GLP-1 medications.

Yu Zhong, Co-founder of Haomai Technology
Yu Zhong pointed out that current R&D directions include:
1) Long-Acting Strategies: The first-generation GLP-1 therapies primarily relied on amino acid sequence modifications; the second generation introduced innovations such as fatty acids, fusion proteins, PEGylation, and microspheres, enabling the groundbreaking achievement of once-weekly dosing. Building on this, the third-generation approaches—featuring antibody-conjugated peptides, antibody-fusion peptides, and atom-layer-deposited coating particles for controlled release—have pushed the envelope further, aiming for an even more extended dosing interval of just once a month. By reducing the frequency of administration, these advancements significantly enhance patient compliance. Additionally, hydrogel-based sustained-release systems and gene therapy are also being explored as promising alternative solutions.
2) New dosage forms and delivery methods: The development of innovative formulations like microneedles and inhalation devices, as well as the creation of oral medications, aims to enhance patients' medication experience and improve treatment adherence.
3) Multi-target approach: In drug design, in addition to targeting GLP-1 itself, researchers are also developing compounds focused on other key targets to enhance efficacy or expand into new therapeutic areas, such as NASH, Alzheimer’s disease, kidney diseases, and heart failure.
4) Combination/Compound Strategies: By combining or formulating with other drugs, these strategies aim to achieve fat-loss and muscle-building effects while minimizing adverse side effects.
At the end of 2023, AstraZeneca secured exclusive rights—comprising an upfront payment of $185 million plus up to $1.825 billion in milestone payments—for the development and commercialization of Chengyi Bio’s ECC5004 outside of China, sparking widespread interest in oral GLP-1 small-molecule therapies. According to Yu Zhong, a series of key events in 2018—including the public release of Sinochem Pharmaceutical’s patent WO2018056453 for Orglifloron, Pfizer’s patent WO2018109607 for Danuglipron, and Eli Lilly’s acquisition of global development and commercialization rights for Sinochem’s OWL833 (Orglifloron) through an upfront payment of $50 million plus milestone payments—marked 2018 as the optimal time to strategically position oneself in the rapidly expanding field of oral GLP-1 small-molecule drugs.
In the second half of 2023, Eli Lilly acquired Versanis' ActRIIA/B monoclonal antibody, Bimagrumab, for $1.925 billion. In 2024, Eli Lilly, Amgen, and others invested $170 million in BioAge Labs to support a Phase 2 trial evaluating the combination of Azelaprag and tirzepatide for obesity treatment—marking the beginning of a global surge in research aimed at addressing the critical clinical challenge of fat loss combined with muscle gain.
Yu Zhong also analyzed the R&D trends of GLP-1 class drugs from the perspective of patent big data. China has witnessed a rapid surge in GLP-1 patents since 2020, surpassing the U.S. by 2023. From the formulation standpoint, both oral and injectable GLP-1 patent applications have their own unique strengths. In terms of target combinations, GLP-1/GIP, GLP-1/GCG, and GLP-1/GCG/GIP continue to maintain high levels of patent activity, while GLP-1/PYY and GLP-1/secretin have seen significant growth in recent years, making them particularly noteworthy. Regarding institutional patent filings, Gasherbrum Bio appears to be a potentially值得关注 new entrant among players that have entered the market in recent years.
At the conclusion of the meeting, the participating experts engaged in an in-depth discussion on the challenges surrounding clinical needs, target discovery, and mechanistic insights. They generally agreed that addressing clinical needs remains the top priority in new drug development. To tackle the hurdles in target discovery and understanding drug mechanisms, the experts recommended leveraging cutting-edge technologies such as genomics and proteomics to uncover promising therapeutic targets for diseases. At the same time, they emphasized the importance of focusing on key factors like target specificity, binding affinity, and drug molecule design—elements crucial for enhancing treatment efficacy while minimizing adverse side effects.
About Shengshi Taikе
Shengshi Taikе is a biotechnology company in the commercialization phase, with its core product, an innovative Class 1 drug called Senglitin (formerly known as Shenglitin), poised for imminent market launch. Founded in 2010 in Suzhou Industrial Park, the company boasts a core team with decades of global experience spanning the entire lifecycle of pharmaceutical products. We are dedicated to the research, development, and industrialization of high-quality, differentiated small-molecule innovative drugs. Leveraging an integrated drug R&D technology platform and a diverse business perspective, we have built a robust pipeline of cutting-edge therapies targeting areas such as diabetes management, cancer treatment, and autoimmune diseases.
