Media Spotlight | Yu Qiang, the "Not-So-Smart" Entrepreneur Behind Senglitin, His Diabetes Drug
Release date:
2025-04-16

· The Phase I trial of senglutide boldly featured a head-to-head comparison with sitagliptin;
· Senglutide has become the first in China's diabetes drug field to receive approval for a "Phase II-to-III clinical trial" pathway, skipping Phase II altogether.
· Yu Qiang has high hopes for CGT-1881, the company’s first China-U.S. dual-regulatory project targeting the CXCR4 antagonist;
· Shengshi Taikang continues to advance its IPO process this year.
Yu Qiang, founder and CEO of Shengshi Taikang, a chemist with a Ph.D. from Peking University, founded a biotech company in the U.S. in the early 2000s before returning to China to establish Shengshi Taikang. Under his leadership, the company has developed a groundbreaking new drug for type 2 diabetes: Senglitin, a next-generation DPP-4 inhibitor that has now been approved for market launch.
Yu Qiang believes his success involved a bit of luck: "Even someone as 'not-so-smart' as me could successfully develop a new drug—so 'smart' entrepreneurs and scientists should definitely be able to do it even better," he said with a smile.
However, entrepreneurship, in his view, is a serious endeavor "that requires solid scientific research and unwavering perseverance." As a medicinal chemist, Yu Qiang takes immense pride in Senglitin, the compound he personally optimized. And clinically speaking, earning the distinction of being the first in the diabetes drug field to advance directly from Phase II to Phase III clinical trials has earned him widespread acclaim.
Fragment Molecules: The Cornerstone of Drug Development
Yu Qiang's connection with diabetes medications dates back to the 1990s, when he studied under Academician Xing Qiyi, a renowned organic chemist and professor in the Department of Chemistry at Peking University. Academician Xing was a pioneering figure in the field of artificial synthesis of bovine insulin—a scientific breakthrough often hailed as one of China’s closest achievements to winning the Nobel Prize.

Dr. Yu Qiang
After earning his PhD in Chemistry from the University of Kansas in the United States, Yu Qiang founded a U.S.-based company in 2006 that specializes in providing fragment molecules for drug discovery and development.
"Fragment molecules, simply put, are the basic building blocks that make up small-molecule drugs," Yu Qiang explained.
In the evolution of small-molecule drugs, targeted therapies have undoubtedly been a revolutionary breakthrough. As research continues to advance, scientists have increasingly discovered that certain enzymes can serve as ideal drug targets. These active targets provide crucial "action spaces" for small-molecule medications. Take diabetes, for instance—DPP-4 is a prime example. By carefully designing a molecular structure that competes with the enzyme’s natural ligand, researchers can effectively disrupt the enzyme’s signaling pathways, ultimately leading to therapeutic benefits.
However, the complexity of living organisms far surpasses what people initially imagine. Enzymes aren’t the neatly shaped structures—like rigid forms—that scientists once thought they were, easily fitting into a single molecule. In reality, an enzyme’s structure more closely resembles a hand: intricate, with complex contours and uneven surfaces. As a result, if you try to design a perfectly shaped molecule to bind to an enzyme, it might only manage to wrap around part of the enzyme—leaving its deeper, critical regions untouched—thus failing to fully inhibit or activate the enzyme’s activity as intended.
“That’s precisely why fragment molecules are especially crucial in drug development—they can serve as the building blocks for creating more complex, highly active small-molecule drugs.” Yu Qiang explained with a metaphor: “Fragment molecules are like words in our poetry—flexibly adaptable, ready to be woven into different verses. Similarly, these fragment molecules can be seamlessly integrated into diverse drug structures.” In fact, Yu Qiang is also known for his talent in writing poetry on a regular basis.
The fragment molecule was the original source of inspiration for sengliptin.
Sigletin leaves room for Yu Qiang.
At the end of 2006, Merck’s first DPP-4 inhibitor, sitagliptin, was approved in the United States for the treatment of type 2 diabetes, sending waves of excitement around the globe. DPP-4 inhibitors work by inhibiting the activity of the DPP-4 enzyme, which enhances and prolongs the effects of GLP-1, thereby stimulating insulin secretion while suppressing glucagon release—and ultimately helping to manage blood sugar levels effectively.
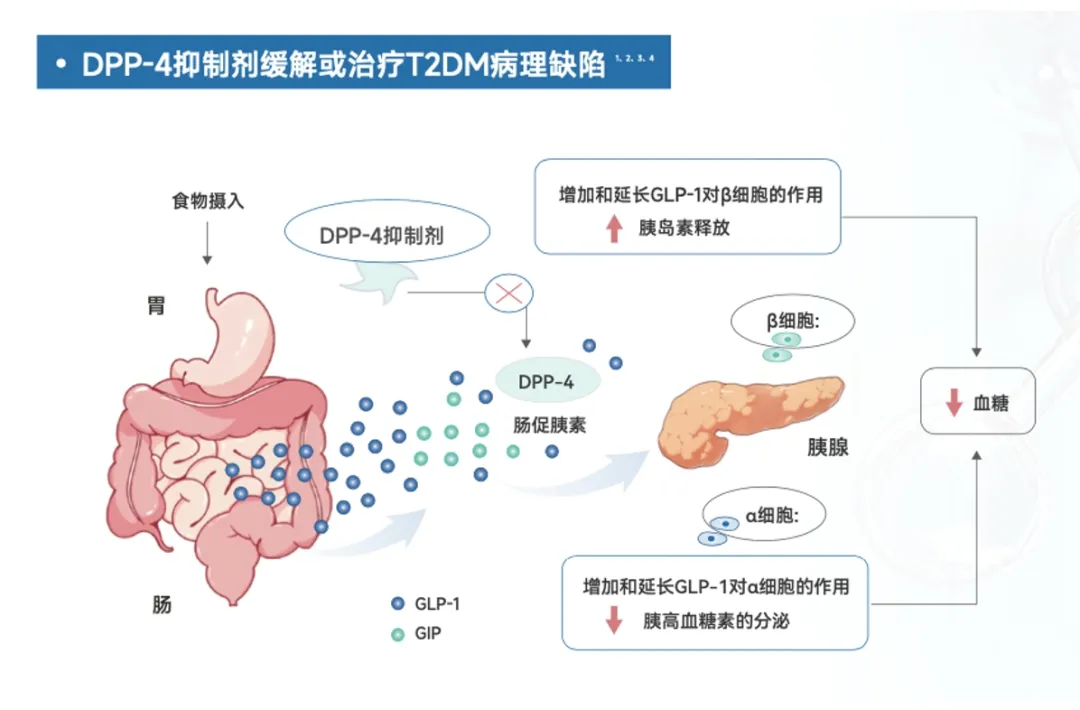
The Mechanism of Action of DPP-4 Inhibitors
Yu Qiang remarked with emotion: "Historically, most targeted drugs have focused primarily on cancer, while in the field of diabetes—the condition affecting the largest number of patients worldwide—neither insulin nor metformin has a clear-cut concept of targeting. Although their blood sugar-lowering mechanisms are effective, the underlying signaling pathways remain unclear. Many companies have attempted to develop new drugs targeting diabetes, but most have ultimately failed."
The DPP-4 enzyme was discovered in 1966, and its target proved to be both clear and highly effective. After four decades of intensive research, it finally emerged in 2006 in the form of sitagliptin. "Drug development is inherently fraught with failure—it typically takes at least two generations of effort," says Yu Qiang. "That’s why the successful creation of the world’s first DPP-4 inhibitor in 2006, a groundbreaking drug with a well-defined mechanism and a precisely targeted approach, has had a profound impact on the diabetes treatment landscape."
At the time, the crystal structure of the DPP-4 enzyme had already been successfully determined, providing crucial insights into the molecular environment after binding to the enzyme. Although AI technologies weren’t as widespread back then as they are today, a method known as Computer-Aided Drug Discovery (CADD) was already helping to accelerate drug development.
During his postdoctoral research, Yu Qiang was already involved in numerous CADD projects. That’s why, when sitagliptin was introduced to the market, he immediately recognized the business opportunity it presented. He carefully analyzed Merck’s marketed product and identified specific areas where it could still be improved—creating a clear opening for Yu Qiang to surpass the competition.
He and his team discovered that the chemical structure of sitagliptin consists of two main components—on one side is a benzene ring carrying three fluorine atoms. Merck had already conducted extensive research on this, publicly sharing their related findings. Building on this knowledge, Yu Qiang and his team shifted their focus to fine-tuning the other side of the molecule: the fused-ring structure containing an amino group, as this part showed clear potential for improvement. Intuitively, he believed these enhancements could open up entirely new possibilities, so he embarked on numerous optimization efforts aimed at refining the molecular architecture.
Yu Qiang decided to dive deep into research by starting with the very foundation of molecular structures. While developing fragment molecules, he adhered to a set of specific standards—criteria that are closely tied to the design of these fragments. First, the design process focuses on carefully crafting interfaces, much like LEGO bricks, which require a variety of connectors to link different components seamlessly. Second, he pays close attention to the physicochemical properties, as this is a critical aspect of fragment-molecule engineering. At the same time, Yu Qiang strives for continuous improvement, such as enhancing the molecule's affinity for the DDP-4 enzyme and other related factors.
After successfully selling a significant number of fragment molecules, Yu Qiang realized that many medicinal chemists were already focusing their research on DPP-4, clearly demonstrating the value of DPP-4 inhibitors. Recognizing this growing trend, he began to envision developing his own drug in this area—and it was then that the idea of starting his own venture first took root. "I’ve always dreamed of bringing a drug all the way through clinical development. For me, that would be the greatest honor of my career."
Returning to China to Found Shengshi Taikang
Between 2008 and 2009, the country began aggressively implementing supportive policies for the biopharmaceutical industry, launching a major national initiative focused on the creation of innovative new drugs. Although the domestic biopharmaceutical sector was still in its early stages at the time, Yu Qiang recognized this historic opportunity and decided to dedicate himself to this promising field.
In 2010, Yu Qiang and his Peking University classmate Ding Juping founded Shengshi Taikang in Suzhou's BioBAY. They synthesized a series of compounds and conducted enzyme activity tests. Initially, with limited funding, they had no choice but to opt for relatively inexpensive testing methods. Surprisingly, however, they discovered that some of the compounds exhibited unexpectedly high levels of activity.
After multiple rounds of refining and optimizing the molecular structure, they finally introduced senglitazone—a next-generation, highly selective DPP-4 inhibitor. What sets it apart is its ability to form a stable co-crystal structure upon binding to DPP-4, a feature that enhances senglitazone's potency while improving its safety profile. As a result, this innovative compound excels in effectively managing blood glucose levels in adult patients with type 2 diabetes.
Yu Qiang explained that this is largely due to the molecular structure of sengliptin, which features an additional methyl group. This unique structural element allows sengliptin to better fit into DPP-4's binding pocket, thereby enhancing the drug's efficiency in interacting with its target.
As a result, Shengshi Taikang has continuously received support from the national "12th Five-Year Plan" and "13th Five-Year Plan" major new drug development initiatives. For the startup company, which at the time had just over ten employees, this was undoubtedly a tremendous validation of its potential.
Pioneering the "DPP-4 'Step II to Step III' without Delay" approach
In the Phase I trial of senglatide, Yu Qiang and his team conducted a head-to-head study comparing it directly with sitagliptin, enrolling not only healthy participants but also patients with type 2 diabetes—a decision that was remarkably bold.
They decided to conduct a large-scale Phase I trial involving approximately 200 participants, which is equivalent to the size typically seen in Phase II clinical trials. Under the guidance of Professor Jiang Ji, a renowned clinical pharmacologist and former director of the Clinical Pharmacology Center at Peking Union Medical College Hospital, six Phase I clinical trials were completed within 18 months. Finally, based on the results of these trials, a quantitative pharmacology model was introduced—and was subsequently developed under Professor Jiang’s mentorship by Dr. Lu Wang and her team from the Phase I Clinical Research Center at Jiangsu Provincial People’s Hospital.
The results show that sengliptin exhibits favorable clinical efficacy, effectively reducing HbA1c even at a 50 mg dose. Its DPP-4 inhibitory activity is comparable to that of 100 mg sitagliptin, while its plasma concentration reaches peak levels more rapidly and maintains a longer half-life, leading to more sustained blood sugar control in steady-state conditions. In terms of safety, sengliptin did not cause any unexpected adverse effects in patients.
Yu Qiang still remembers that night vividly—after his research data turned out exceptionally impressive, Professor Jiang Ji excitedly called Ding Juping, the company’s co-founder, late at night and exclaimed: "I’ve never seen a domestically produced drug in this field deliver such outstanding results!"
In October 2019, based on promising Phase I trial data and a robust PK/PD model, senglitin received approval from the CDE to skip Phase II clinical trials altogether and proceed directly to Phase III, pioneering the "Phase II-to-III bypass" approach for DPP-4 inhibitors. According to Yu Qiang, the CDE’s decision was grounded in two key factors: first, senglitin’s superior Phase I clinical data; and second, the successful validation of the quantitative pharmacology model using patient data, with the model’s predictions closely aligning with actual outcomes.
The Phase III clinical trial, led by Professor Li-Nong Ji, a renowned diabetes expert from Peking Union Medical College Hospital, has launched two pivotal studies in China, enrolling approximately 1,000 type 2 diabetes patients in total. These trials aim to evaluate the efficacy and safety of senglatide as monotherapy, as well as its combination with metformin, for the treatment of type 2 diabetes.

Professor Ji Linong delivered a speech at the 2023 Peking University Diabetes Forum.
The results showed that, in monotherapy, sitagliptin 50 mg reduced patients' HbA1c levels by 1.08%. After 24 weeks of treatment, 45% of patients achieved an HbA1c level below 7%, which was 3.5 times higher than the placebo group. Additionally, the proportion of patients with HbA1c below 6.5% was 10 times greater compared to the placebo group. For patients whose monotherapy with metformin alone was insufficient, adding sitagliptin resulted in a further significant reduction in HbA1c—by as much as 1.23%. By week 24, the percentage of patients achieving an HbA1c below 7% climbed to 51%, representing 3.5 times the rate observed with metformin alone. Moreover, the proportion of patients with HbA1c below 6.5% was 5.5 times higher than with metformin monotherapy alone.
In the Phase III trial, they also encountered several significant challenges. For instance, recruiting participants and managing costs proved to be major headaches. However, to demonstrate the advantages of sengliptin, they decided to conduct the trial simultaneously with both a low-dose group (50 mg) and a high-dose group (100 mg). Moreover, during the subsequent safety study, all patients originally assigned to the low-dose group were switched over to the high-dose group to further evaluate safety. This was yet another bold move—but Yu Qiang and his team remained steadfast in their confidence in their research and their own judgment.
Ultimately, their efforts paid off. The results showed that the 50 mg low-dose sengliptin group demonstrated significant efficacy in reducing HbA1c levels, with even greater reductions observed in patients who initially had higher blood glucose levels. In addition to its outstanding blood sugar-lowering effects, sengliptin also exhibited a safety profile comparable to the placebo group in the high-dose 100 mg group during the subsequent 28-week period, effectively addressing the common adverse reactions often associated with existing marketed products.
During the approval waiting period, the Center for Drug and Food Supervision and Inspection under the National Medical Products Administration conducted on-site inspections of the research centers involved in Sengletin’s two clinical trials—and both passed with high quality, much to their relief. "This clearly demonstrates that our research is solid and our data is reliable," Yu Qiang said proudly.
Willing to be the stepping stone for China's innovative medicines.
Yu Qiang said that while Sengretin's success did involve a bit of luck, solid scientific research was absolutely essential. He emphasized that every step of the scientific process must be conducted with meticulous rigor—otherwise, challenges are likely to arise down the road. At the same time, perseverance remains the key to achieving success.
In 2015, Shengshi Taikang faced a severe funding crunch. To weather the crisis, they were forced to sell one of their pipeline products currently in development. In addition to "selling off" their own "baby," they also raised capital by selling pharmaceutical raw material intermediates—and even the management team personally contributed their own money to keep the company afloat.
"Hopefully, the government and investors can understand the challenges of innovation in the pharmaceutical industry," Yu Qiang said.
2025 marks the 15th year since Shengshi Taike Innovation was founded, and it will also be a pivotal year for the commercial launch of sengliptin—particularly as the company prepares to navigate critical health insurance negotiations. Yu Qiang emphasizes that these negotiations should strike a balance: ensuring affordability for patients while fully accounting for the high-quality standards and significant clinical benefits that innovative drugs like sengliptin can deliver.
Currently, metformin combined with GLP-1 receptor agonists has become the primary treatment approach in clinical practice for type 2 diabetes. According to a report by Sullivan Research, by 2030, GLP-1 receptor agonist drugs in China are expected to surpass a market size of 30 billion yuan, highlighting their promising future prospects.
In 2025, the company will also continue advancing its IPO process in the capital markets. With the support of capital, Shengshi Taikang will accelerate the comprehensive commercialization of senglatin and swiftly push forward its pipeline of future innovations, enabling the company to achieve a transformative "from point to area" growth trajectory.
Now, Shengshi Taikang has grown to a certain scale, but Yu Qiang still has an unfinished dream.
"I'd like to develop another new drug, proving that our success wasn’t just a coincidence," Yu Qiang said with a smile. He’s currently excited about CXCR4 antagonist CGT-1881, a project that has nearly completed its Phase I clinical trials. Clinical data so far indicate the compound shows remarkable promise—particularly in stem cell mobilization and for treating WHIM syndrome. This drug also marks the company’s first-ever New Drug Application submitted simultaneously in both China and the U.S., opening up broad potential applications and significant market value in areas such as malignant tumor treatment, stem cell mobilization, immune disorders, and genetic diseases.
Finally, he expressed his hope that more founders would dedicate themselves to the cause of new drug development and remain committed to it. "I am a stepping stone for China’s innovative medicines—I’m willing to pave the path of hope toward a healthier future for China." In conclusion, we encourage all new drug researchers with a poem by Dr. Yu:
Each corner holds heavenly melodies,
Liesha ascends to the hall of fame.
Morning light shines on Yunting,
A prosperous era greets the rising sun.
