Milestone | Shengshi Taikang’s Next-Generation DPP-4 Inhibitor Approved for Market
Release date:
2024-12-05

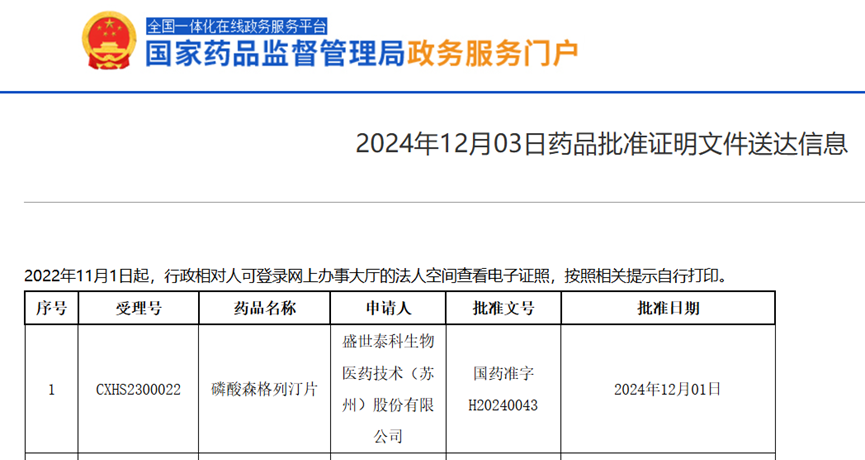
Today, the official website of China's National Medical Products Administration (NMPA) announced that Shengshi Taikang's independently developed Class 1 innovative drug, Sengreglin Phosphate Tablets (formerly known as: Phosphate Sitagliptin Tablets It has been approved for launch in China. This It's a next-generation, highly selective DPP-4 inhibitor , Suitable for improving blood glucose control in adults with type 2 diabetes.
According to the "Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes," the prevalence of diabetes among adults in China has risen to 11.2%, with over 90% of cases being type 2 diabetes. To address the significant clinical demand, several new drugs with mechanisms distinct from traditional oral antidiabetic medications have emerged in recent years—among them, dipeptidyl peptidase-4 (DPP-4) inhibitors stand out. These innovative therapies not only help improve hyperglycemia but also carry a lower risk of causing hypoglycemia or weight gain. With their rapid oral absorption, long half-life, and sustained efficacy, DPP-4 inhibitors are often referred to as "smart" glucose-lowering agents and have already earned widespread recommendations in both domestic and international treatment guidelines.
Sotagliflozin, a next-generation, highly selective DPP-4 inhibitor independently developed by Shengshi Taikang, was consecutively selected for China’s 12th and 13th Five-Year Plans’ “Major New Drug Development” initiatives. Thanks to its outstanding performance in a head-to-head Phase 1 clinical trial compared to the market-leading product, the drug was granted an exemption from Phase 2, allowing it to advance directly into Phase 3 clinical trials—paving the way for a groundbreaking "Phase 2-free, Phase 3-ready" approach in clinical testing within this therapeutic area.

The results of the Phase 3 clinical trial of senglatagliptin, led by Professor Ji Linong, Director of the Department of Endocrinology at Peking University People's Hospital and Director of the Peking University Diabetes Center, showed that in the monotherapy trial of senglatagliptin: At the end of week 24, the 50mg and 100mg dose groups showed HbA1c reductions of 1.08% and 1.07%, respectively. In the sitagliptin plus metformin treatment trial, the 50mg and 100mg dose groups achieved HbA1c reductions of 1.23% and 1.17%, respectively, by week 24. Meanwhile, both sets of experimental data showed that patients with higher baseline blood glucose levels experienced greater reductions in HbA1c.
In addition to its excellent blood sugar-lowering effects, what makes Senglitin even more remarkable is that, in the 100mg dose group (high-dose group) compared to the placebo group during the subsequent 28 weeks, the incidence of adverse reactions was similar to that of the placebo group, further demonstrating its safety and addressing the common side effects often seen with currently available products.
Currently, metformin combined with GLP-1 receptor agonists has become the primary treatment regimen in clinical practice for type 2 diabetes. According to a Sullivan research report, by 2030, GLP-1 receptor agonist drugs are expected to surpass a market size of 30 billion yuan in China, highlighting their promising and expansive growth prospects.

Ding Juping, Co-founder and President of Shengshi Taikang, said: "We are thrilled to see our company's independently developed product—Senglitin Phosphate Tablets—approved for market launch after a decade of dedicated research. This drug delivers twice the therapeutic efficacy with half the dosage, positioning it as the best-in-class new medication for blood sugar control. We sincerely hope that once approved, it will soon benefit the vast number of diabetes patients across China."
Dr. Yu Qiang, Founder and CEO of Shengshi Taike, stated: "The smooth approval and launch of Senglitin are a testament to Suzhou and its thriving entrepreneurial ecosystem, as well as the strong support from numerous government departments. This milestone also reflects our commitment to repaying Suzhou's business-friendly services with the 'unwavering promise' embedded in our innovative drug. At this very moment, I’d like to celebrate the successful market debut of this groundbreaking new medication with an original poem." ”
Senmu Ge Heavenly melody,
Column Cai Deng Hall.
Morning light shines on the clouds. Ting ,
Prosperous Era Welcome Chaoyang 。
