Shengshi Taikang: Trading "A Promise Worth a Thousand Pieces of Gold" for "Dreams Come True"
Release date:
2025-01-07

As Shengshi Taikang Biopharmaceutical Technology (Suzhou) Co., Ltd. celebrates 15 years of扎根 in Suzhou Industrial Park, the company has reached a remarkable milestone: its first-in-class, independently developed innovative drug—Sengludine Phosphate Tablets—has been approved for launch in China. Reflecting on this achievement, Yu Qiang, Founder and CEO of Shengshi Taikang, penned a heartfelt poem: "Amidst forests, celestial melodies unfold; Colors converge as they ascend to the grand hall. Morning light bathes the tranquil shore, heralding a new era under the rising sun."

"China is a global leader in diabetes, boasting the largest number of diabetic patients worldwide. Back in 2008, I heard news that the country was beginning to prioritize the biopharmaceutical industry. By around 2010, I was determined to return home—equipped with my early-stage research and development achievements—to launch a Class 1 innovative drug for diabetes treatment, funding it entirely on my own and assembling a dedicated team." Recalling more than a decade ago, Yu Qiang still remembers it vividly. "Deciding to come back and choosing to start my business right here in the park was ultimately influenced by the thoughtful, professional responses I received during our ongoing email exchanges. At the time, I was still based in the U.S., reaching out directly to the park’s government via email with questions about entrepreneurship. The replies I got weren’t just impeccably written in fluent English—they were also remarkably knowledgeable and expertly crafted. Looking back, those initial interactions have clearly proven that making this decision was the right choice."
The original passion for entrepreneurship and the bold spirit of innovation have now come together, creating a heartwarming story of mutual pursuit between the park and its talented individuals. "The smooth approval and launch of Senglitin were made possible thanks to Suzhou and the park—a fertile ground for startups—as well as the strong support from numerous departments," said Yu Qiang. "This is also our way of repaying the park’s exceptional business-friendly services with the ‘unwavering commitment’ that defines our innovative drug development."
Since establishing itself in the park in 2010, Shengshi Taikang has been rooted in Suzhou while maintaining a global outlook, focusing exclusively on the research and development and production of innovative small-molecule drugs. Leveraging an integrated drug R&D technology platform and a diversified business vision, the company has built a robust pipeline of cutting-edge therapies spanning areas such as diabetes management, cancer treatment, and autoimmune diseases.
Funding—
"The First Capital" to Leverage Innovation
In 2010, armed with extensive scientific research and practical experience in pharmaceutical R&D, Yu Qiang, along with his partner Ding Juping, founded Shengshi Taikang at Suzhou Bio-Medical Industrial Park (BioBAY), focusing on the development and commercialization of innovative small-molecule drugs aimed at delivering breakthrough therapies.
"China is a global leader in diabetes, with the largest number of diabetic patients worldwide. Back in 2008, I heard news that the country was starting to prioritize the biopharmaceutical industry. By around 2010, I was determined to return home—armed with my early-stage research and development成果—to launch a Class 1 innovative drug for diabetes treatment, funding it myself and assembling a dedicated team." Recalling more than a decade ago, Yu Qiang still remembers it vividly. "Deciding to come back and choosing to start my business right here in the park was ultimately influenced by the thoughtful, professional responses I received in our ongoing email exchanges. At the time, I was still based in the U.S., reaching out directly to the park’s government via email with questions about entrepreneurship. The replies I got weren’t just impeccably written in fluent English—they were also remarkably knowledgeable and expertly crafted. Looking back, those initial interactions have clearly proven that making this decision was the right choice."

"Everything is difficult at the beginning—even though the conditions for starting a business back in China are relatively favorable. For a fledgling biopharmaceutical R&D company in its early stages, the challenges are easy to imagine, with funding shortages being the first major hurdle. Fortunately, the park has provided exceptionally generous financial support to leading talent," said Yu Qiang. "When we first settled here in 2010, the park offered 'big-ticket' incentives worth up to 10 million yuan to top-tier professionals in the biomedicine sector. These benefits included housing subsidies, rent reductions, interest-rate-subsidized loans, and even seed funding. More importantly, Yuanhe Holdings—formerly known as Sino-Singapore Venture Capital—stepped in with an angel investment of 6.5 million yuan, which proved to be a much-needed lifeline for our startup."
Later, the company’s new drugs were successively selected for China’s national “12th Five-Year Plan,” “13th Five-Year Plan,” and the “Major New Drug Development” special projects, placing them firmly on the government’s key support track—and securing additional funding as a result. Meanwhile, both the park’s Enterprise Development Service Center’s guidance fund and leading venture capital firms provided crucial financial backing at various stages of Shengshitaike’s growth. Under the guidance of the government’s industrial fund, these investments also attracted more private capital to join in, collectively fueling the company’s innovation efforts. “As I’ve always believed, it was the park that provided the ‘1’—and only then did private investors step in to add all those ‘0s’ afterward,” Yu Qiang said with a smile.
Funding has fueled corporate growth, which in turn has driven industry development. Currently, Shengshi Taikang has established a robust pipeline of drug candidates, spanning from pre-clinical stages all the way to commercialization—and this diverse portfolio is strategically positioned for sustained advancement. Notably, several of its R&D projects have already secured U.S. clinical approvals, marking a significant milestone as the company takes a confident step toward bringing innovative medicines to global markets.
Talent—
A "powerful engine" driving faster innovation supply
Talent is the foundation upon which businesses rely for survival, growth, and expansion. After more than a decade of dedicated effort, Shengshi Taikang has not only achieved a "grand slam" of science and technology projects—from national to local levels—but has also secured a "talent grand slam," seamlessly integrating talent development with cutting-edge innovation. This has created a dynamic synergy where talent and technological advancements advance side by side.

At the end of 2021, the park took the lead in China by launching a pilot program for evaluating professional titles in biopharmaceutical engineering, marking the first time that this field secured a formal place within the country’s professional title system. Among the initial group of professionals approved in the review process, Shengshi Taikang had 16 individuals recognized, with 12 of them earning senior-level titles in biopharmaceutical engineering. Yu Qiang, who serves as one of the chairpersons of Suzhou’s Senior Professional and Technical Qualification Review Committee for Biopharmaceutical Engineering, personally witnessed the entire evaluation process. In his view, if companies can identify and select a cohort of highly qualified talent from a practical standpoint—and then leverage these individuals to forge connections with universities or research institutions—this "talent bridge" could pave the way for future collaborations between academia and industry. Together, they could jointly develop tailored training programs designed to meet the needs of the biotech sector, ultimately creating a powerful "magnetic field" that attracts and retains top talent.
The park fully considers the needs of innovative and entrepreneurial talent, not only by fostering a superior industrial environment but also by continuously enhancing the quality of life for residents. To date, Shengshi Taikang has steadily built a comprehensive talent pipeline spanning the entire industry chain—from R&D to commercialization—while maintaining remarkable stability within its workforce. "Integrity, Innovation, Pragmatism, and Service to Society" is Shengshi Taikang’s cherished "eight-character motto"—a guiding principle that underscores the importance of upholding high standards, nurturing continuous innovation, embracing an authentic pursuit of truth, and staying true to our mission of benefiting society. Only by adhering to these values can we confidently navigate challenges and ultimately achieve our goals.
Innovation has always been the guiding principle behind Shengshi Taikang’s development. According to Yu Qiang, for a startup to achieve sustained growth, its core lies in talent, while the driving force behind that talent is innovation. That’s why Shengshi Taikang strives to ensure its pipeline products offer distinct advantages over existing clinical treatments—and when targeting the same therapeutic area, these products aim to deliver both superior quality and meaningful differentiation. "In 2012, our team already developed an early version of sengliflozin, but its technical performance was only on par with comparable imported products available on the market. We’re here precisely because of our commitment to innovation. Without breakthroughs that surpass market standards, we wouldn’t truly be making progress, and we’d certainly fail to live up to the support and trust shown by governments at all levels."
So, we resolutely scrapped our original R&D plan and started over. By the end of 2016, perseverance finally paid off—Sengliti, a superior-performing drug, was finally launched, prompting us to submit its clinical application to the National Medical Products Administration. "Sengliti not only effectively manages high blood sugar but also minimizes the risk of hypoglycemia and weight gain. With its rapid oral absorption, long half-life, and sustained efficacy, it delivers "more bang for your buck," earning it the nickname "smart" diabetes medication."
Industry —
Connecting the "last mile" of the innovation ecosystem
Stick to the path of innovation—ten years of sharpening one sword.
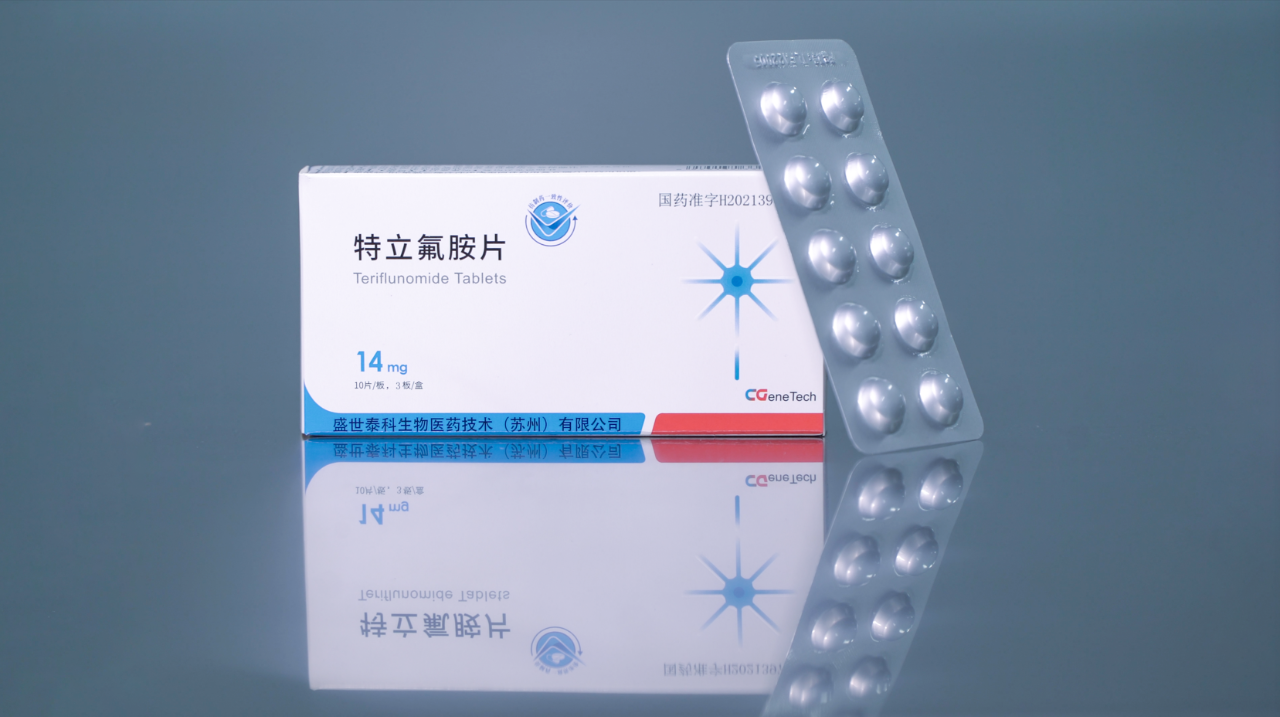
In August 2022, Shengshitai Research & Development launched the first domestically produced teriflunomide tablet for sale, offering a new treatment option and ensuring drug supply for multiple sclerosis (MS) patients in China. This milestone also marks the company's official entry into its commercialization phase.
“Over the past decade and a half of entrepreneurship in the park, I’ve witnessed the most significant transformation in the园区’s industrial development: the biopharmaceutical sector has grown from scratch to become one of China’s leading innovation hubs. Not only has its scale expanded dramatically—from just over 100 companies back then to more than 1,000 today—but it has also fostered a comprehensive ecosystem that brings together entrepreneurs, top-tier talent, research institutions, and venture capital firms. This has created a powerful cluster advantage, enabling seamless upstream-downstream collaboration and driving collaborative innovation across the industry.” Looking back on those years, Yu Qiang reflects, “Passion is a force”—the very same passion that first inspired him to embark on this entrepreneurial journey. At its core, his vision remains unchanged: he hopes that domestic patients will soon have access to high-quality, cutting-edge, domestically produced medicines that truly make a difference.

Pharmaceutical development, especially the research and development of innovative drugs, is a lengthy and capital-intensive process. From the lab all the way to market launch, therapies like teriflunomide and sotagliflozin have already successfully navigated the "last mile," effectively completing Shengshi Taikang's "innovation chain" and "innovation pathway." This milestone not only validates the effectiveness of the company's meticulously designed innovation platform but also signals its ability to sustainably develop new drugs in the future.
Currently, the company is actively preparing for the commercialization of senglatide by continuously expanding its market presence, ensuring that high-quality products reach a broader patient population. Meanwhile, Shengshi Taikang is also developing multiple projects in the fields of diabetes and oncology, committed to delivering more innovative and effective treatment options that meet patients' needs—and ultimately setting a new industry standard.
“Over the past decade and a half of entrepreneurship in the park, I’ve witnessed the most significant transformation in the园区’s industrial development: the biopharmaceutical sector has grown from scratch to become one of China’s leading innovation hubs. Not only has its scale expanded dramatically—from just over 100 companies back then to more than 1,000 today—but it has also fostered a comprehensive ecosystem that brings together entrepreneurs, top-tier talent, research institutions, and venture capital firms. This has created a powerful cluster advantage, enabling seamless upstream-downstream collaboration and driving collaborative innovation across the industry.” Looking back on those years, Yu Qiang reflects, “Passion is a force”—the very same passion that first inspired him to embark on this entrepreneurial journey. At its core, his vision remains unchanged: he hopes that domestic patients will soon have access to high-quality, cutting-edge, domestically produced medicines that truly make a difference.
After 30 years, the park is setting off on a new journey, and Shengshi Taikang’s path of innovation and entrepreneurship will also reach a new milestone. Moving forward, the company will continue to drive breakthroughs from the very source, addressing clinical needs and ultimately benefiting patients worldwide—while helping the park’s biopharmaceutical industry grow stronger and more robust.
