Expert Interview | A Decade of Crafting the Yitian Sword: Dr. Yu Qiang Shares the Story Behind Senglitin Development
Release date:
2025-02-24
Editor's Note:
Recently, the field of diabetes treatment drugs welcomed a new addition—Senegretin, a next-generation, highly selective DPP-4 inhibitor developed by Shengshi Taikang. As the pioneering leader behind this innovative drug, Dr. Yu Qiang, founder of Shengshi Taikang, has spent over a decade crafting a remarkable story of perseverance and breakthrough in pharmaceutical research and development.
Written by | Mao Donglei
Yu Qiang's journey into diabetes drug research dates back to his student days at Peking University's Department of Chemistry. It was during this time that he studied under Academician Xing Qiyi, a founding figure in Chinese organic chemistry—and the very scientist who led the groundbreaking effort to artificially synthesize bovine insulin, an achievement often hailed as one of China's closest scientific breakthroughs to winning the Nobel Prize. Under Academician Xing's mentorship, Yu Qiang developed a deep passion for advancing diabetes drug development.
After graduating from Peking University, Yu Qiang went to the U.S. for advanced studies, earning his Ph.D. in Chemistry from the University of Kansas. In 2006, he founded a company in the United States, specializing in providing fragment-based molecules for drug discovery and development. When he decided to return to China to start his own business in 2010, he chose the field of metabolism, determined to develop an innovative drug that would deliver significant clinical benefits to diabetes patients in China.

Dr. Yu Qiang, Founder and CEO of Shengshi Taike
Sengliptin breaks through traditional DPP-4 inhibitors
DPP-4 inhibitors are an important class of drugs for treating diabetes, and since Merck introduced sitagliptin—the world's first DPP-4 inhibitor—in 2006, they have become a popular target in the development of new diabetes therapies.
Yu Qiang vividly described DPP-4 inhibitors as the "blood sugar guardians" within the human body for diabetes patients. Inside our bodies, there’s an enzyme called DPP-4—a sort of magical wand—that plays a key role in regulating two crucial signaling molecules essential for lowering blood glucose: GLP-1 and GIP. Normally, these two messengers send signals to the body, instructing it to release insulin promptly when blood sugar levels rise. However, if the DPP-4 enzyme interferes at the wrong moment by breaking down these messengers too quickly, the body fails to receive the necessary signals to lower blood sugar effectively. That’s where DPP-4 inhibitors come in—they act as vigilant guardians, keeping the "magic wand" under control. By inhibiting the DPP-4 enzyme, they prevent it from degrading GLP-1 and GIP, ensuring that these vital signaling molecules can continue delivering their blood-sugar-lowering messages unimpeded. As a result, the body is better equipped to release insulin in a timely manner, helping to keep blood glucose levels in check.
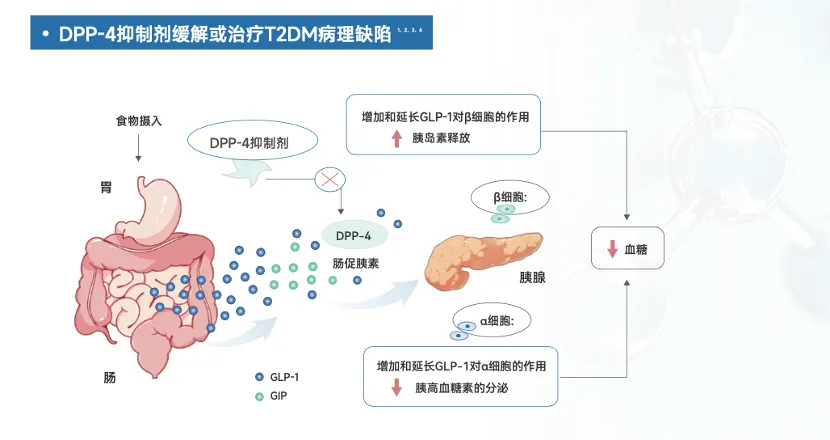
The Mechanism of Action of DPP-4 Inhibitors
Around 2013, several imported DPP-4 inhibitor drugs were successively launched in the Chinese market, simultaneously opening up a massive domestic market for these medications. As these drugs gained traction through medical promotion, more and more doctors and patients became aware of the therapeutic advantages offered by DPP-4 inhibitors—prompting domestic pharmaceutical companies to ramp up their own development efforts. Yu Qiang recalls that at the time, over 10 Chinese pharmaceutical firms had already advanced their respective domestically produced DPP-4 inhibitor candidates into clinical trial stages. In such a fiercely competitive landscape, he firmly believes that only continuous innovation can help companies stand out from the crowd.
Yu Qiang's entrepreneurial and R&D journey began when he founded a company in the U.S., where he meticulously analyzed the DPP-4 inhibitor drug market and studied existing similar medications. He discovered that, although the drugs already on the market had shown promising clinical efficacy, they still had certain limitations—such as challenges in optimizing both therapeutic effects and managing adverse reactions. Fueled by this insight, he decided to tackle the issue at its very root: by examining the molecular structures of these inhibitors to develop a superior DPP-4 inhibitor from scratch.
After numerous attempts, Yu Qiang finally identified a path for improvement based on the molecular structures of other glitazone compounds, resulting in a new compound with enhanced DPP-4 inhibitory activity. Building on this breakthrough, he leveraged his company’s cutting-edge platform—developed abroad at the time—to design over 20 tailored DPP-4 inhibitor fragment molecules (precursor compounds), which were subsequently sold commercially. These fragment molecules have since inspired leading international researchers with fresh approaches to developing next-generation DPP-4 inhibitors, while Yu Qiang himself continues to draw innovative ideas from valuable clinical feedback.
However, making money from selling fragment molecules wasn’t Yu Qiang’s ultimate goal—his real concern was which of these fragments had the highest potential to become a key component of the next-generation DPP-4 inhibitor. Yu Qiang identified the structurally optimal DPP-4 inhibitor compound under the given conditions: the very precursor to senglitin.
However, there’s still a long way to go—from precursor compounds to lead candidates, and then through preclinical and clinical studies. Yu Qiang knows full well that he can’t do it alone; he needs an experienced partner. That’s why he reached out to Ding Juping, his former classmate from Peking University, who has independently led dozens of successful national-level new drug development and regulatory submissions.
With the combined efforts of the two teams, preclinical research on senglitin progressed rapidly. Yu Qiang explained that they conducted a head-to-head comparison study between senglitin and the currently available best-in-class product right in Phase I clinical trials, evaluating multiple aspects such as efficacy, safety, pharmacokinetics, toxicology, and pathology. The results showed that senglitin outperformed the competitor across all key metrics. This outcome has only strengthened Yu Qiang's confidence in senglitin even further.
Sengliptin Clinical Trial Advances "From Phase 2 to Phase 3 Without Delay"
In early 2018, Shengshi Taikang initiated Phase I clinical trials for senglitin. Over the course of 18 months, they successfully completed five Phase I studies, thoroughly evaluating senglitin’s safety, tolerability, and pharmacokinetic/pharmacodynamic profiles. The trial results demonstrated that senglitin exhibits a favorable safety profile and promising pharmacokinetic characteristics. Specifically, its DPP-4 inhibitory activity effectively reduces glycated hemoglobin even at doses as low as 50 mg. Moreover, senglitin reaches peak plasma concentrations within 1 to 2 hours after administration and boasts a longer half-life, enabling sustained, stable blood sugar-lowering effects over time.
In terms of safety, sitagliptin has shown particularly impressive results. Yu Qiang noted that during the Phase I clinical trial, no adverse effects on patients' health were observed with sitagliptin. For diabetic patients who require long-term medication, this is undoubtedly great news.
Thanks to Senglitin's outstanding performance in preclinical studies and Phase I clinical trials, the drug received formal approval from the National Medical Products Administration Center in September 2019, allowing it to skip Phase II and proceed directly to Phase III trials. This decision not only set a precedent for "skipping Phase II and moving straight to Phase III" in DPP-4 inhibitor clinical trials but also laid a solid foundation for Senglitin's accelerated market launch.
Sengliptin monotherapy can improve the HbA1c达标 rate in patients with T2DM.

Professor Ji Linong delivered a speech at the 2023 Peking University Diabetes Forum.
In addition to its excellent blood sugar-lowering effects, what makes Senglitin even more remarkable is that, in the 100mg dose group (the high-dose group) compared to the placebo group over the subsequent 28 weeks, the incidence of adverse reactions was similar to that of the placebo group, further demonstrating its safety and addressing the common side effects often seen with currently available products.
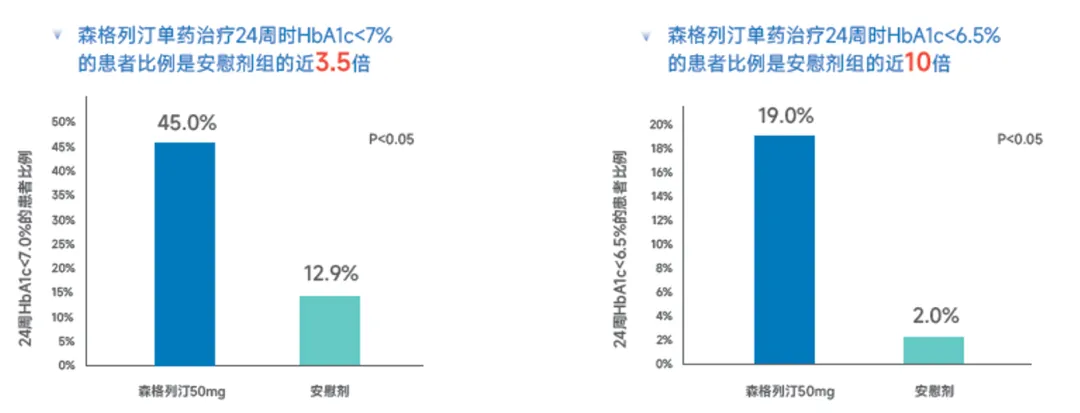
Sengliptin monotherapy can improve the HbA1c达标 rate in patients with T2DM.
This trial was conducted during the challenging times of the pandemic. Yu Qiang still vividly recalls several unforgettable stories that unfolded as they recruited patients and advanced the research. During the Phase III clinical trial, disruptions caused by the pandemic prevented some regional research centers from receiving timely drug deliveries, leaving many participants struggling to access their study medications on schedule.
To address this, the company’s clinical researchers swiftly mobilized, reaching out separately to research centers in other cities and arranging for cold-chain logistics to deliver the medications to these affected areas. They then employed a "human relay" approach, personally handing over the clinical drugs directly to the participants. These concerted efforts not only ensured uninterrupted treatment for the subjects but also yielded exceptional Phase III clinical trial data.
During the trial, some patients lived in villages or communities that were under pandemic restrictions—allowing people to enter but not leave—making it impossible for them to travel to the hospital for follow-up visits. Faced with this challenging situation, researchers, research nurses, and CRCs stepped up to the plate. They visited patients at home to carefully monitor vital signs, physical condition, blood sugar levels, and more. At the same time, they took detailed notes on patients' symptoms, medication use, and overall health status. All of these efforts were aimed at ensuring that patients could continue their follow-ups as prescribed by the trial protocol—ultimately safeguarding both their well-being and the smooth progression of the study.
He also mentioned that, during the trial, it’s essential to enter the collected data into the EDC system on time and to regularly monitor the entered EDC data. However, CRCs/CRAs are often restricted to individual research centers, leaving them with limited workspace—frequently forced to work squeezed into corridors, hunched over a small stool. Despite these challenging conditions, they continue to do their best to ensure timely data entry and thorough monitoring, thereby maintaining the accuracy and reliability of the trial data.
Saxagliptin Approved for Market Launch and Future Blueprint
After a rigorous review process, on December 1, 2024, the marketing application for senglatide finally received formal approval from the National Medical Products Administration for the treatment of type 2 diabetes. At that moment, Yu Qiang felt an overwhelming sense of excitement he could hardly put into words. He recalled: "That was the moment I finally saw the fruits of our years of hard work. Drug development requires patience, perseverance, and unwavering determination. For instance, it took nearly 40 years—from the discovery of the DPP-4 protein to the successful development of the first targeted drug targeting it. But when I witnessed senglatide receiving approval and being able to bring real relief to diabetes patients, I knew every single effort had been worth it."
Yu Qiang also expressed his gratitude to the domestic clinical experts and every single member of the team, noting that it was their collective efforts and dedication that made the successful launch of sengliflozin possible. He particularly thanked the Center for Drug Evaluation under the National Medical Products Administration for their openness and professionalism, which have created an exceptionally supportive environment for biopharmaceutical innovation in China.
By 2030, DPP-4 inhibitors are projected to surpass a market size of 30 billion yuan in China. Yu Qiang noted that looking ahead, 2025 will be a pivotal year for the commercial launch of sengagliptin, with one of the key challenges being negotiations with health insurance providers. He expressed hope that these negotiations will strike a balance—taking into account patients' affordability while also recognizing the quality and clinical benefits of innovative drugs. After all, high-quality new medications deserve adequate profit margins to sustain the ongoing development of innovative pharmaceutical companies, ensuring that patients continue to reap long-term advantages.
Meanwhile, Yu Qiang also revealed that the company will continue advancing its IPO process in the capital markets in 2025, completing the final pre-IPO funding round. With the support of capital, Shengshi Taikang will accelerate the commercialization of sengliflozin and further drive forward its innovative pipeline, enabling the company to achieve growth that expands "from a single point to a broader scope."
The story of sotagliflozin's development is a decade-long journey penned by Dr. Yu Qiang and his team—marked by innovation, breakthroughs, perseverance, optimism, and, above all, heartfelt inspiration. To conclude, Dr. Yu shares a poem dedicated to everyone tirelessly striving on the path of new drug discovery, offering encouragement to all along the way. (Source: Clinical Research Promotion Public Welfare Fund)
Each corner holds heavenly melodies,
Lieshaideng steps into the hall of honor.
Morning light shines on the cloud-strewn shore,
A prosperous era welcomes the rising sun.
—— Yu Qiang
